If a Galvanic Cell Is Prepared Correctly the Initial Multimeter Reading Be
2.1: Galvanic Cells
- Page ID
- 48478
Learning Objectives
- To understand the basics of voltaic cells
- To connect voltage from a voltaic prison cell to underlying redox chemical science
In any electrochemical process, electrons flow from one chemical substance to another, driven by an oxidation–reduction (redox) reaction. A redox reaction occurs when electrons are transferred from a substance that is oxidized to one that is being reduced. The reductant is the substance that loses electrons and is oxidized in the process; the oxidant is the species that gains electrons and is reduced in the process. The associated potential energy is determined by the potential deviation between the valence electrons in atoms of unlike elements.
Because it is impossible to have a reduction without an oxidation and vice versa, a redox reaction can be described as two half-reactions, one representing the oxidation process and i the reduction process. For the reaction of zinc with bromine, the overall chemical reaction is as follows:
\[\ce{Zn(s) + Br2(aq) \rightarrow Zn^{2+} (aq) + 2Br^{−} (aq)} \nonumber\]
The one-half-reactions are as follows:
reduction one-half-reaction:
\[\ce{Br2 (aq) + 2e^{−} \rightarrow 2Br^{−} (aq)} \nonumber\]
oxidation one-half-reaction:
\[\ce{Zn (s) \rightarrow Zn^{2+} (aq) + 2e^{−} }\nonumber\]
Each half-reaction is written to show what is actually occurring in the organisation; \(\ce{Zn}\) is the reductant in this reaction (it loses electrons), and \(\ce{Br2}\) is the oxidant (information technology gains electrons). Adding the two half-reactions gives the overall chemical reaction (Equation \(\PageIndex{1}\)). A redox reaction is counterbalanced when the number of electrons lost past the reductant equals the number of electrons gained by the oxidant. Like whatsoever balanced chemical equation, the overall process is electrically neutral; that is, the net charge is the aforementioned on both sides of the equation.
In any redox reaction, the number of electrons lost by the oxidation reaction(southward) equals the number of electrons gained by the reduction reaction(due south).
In most of our discussions of chemical reactions, we have assumed that the reactants are in intimate physical contact with one some other. Acid–base reactions, for example, are unremarkably carried out with the acrid and the base dispersed in a single phase, such every bit a liquid solution. With redox reactions, however, it is possible to physically split up the oxidation and reduction half-reactions in infinite, as long every bit there is a complete circuit, including an external electrical connection, such as a wire, between the two one-half-reactions. As the reaction progresses, the electrons flow from the reductant to the oxidant over this electrical connexion, producing an electric electric current that can be used to do work. An appliance that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to bulldoze a nonspontaneous redox reaction is called an electrochemical jail cell.
At that place are ii types of electrochemical cells: galvanic cells and electrolytic cells. Galvanic cells are named for the Italian physicist and physician Luigi Galvani (1737–1798), who observed that dissected frog leg muscles twitched when a small electrical stupor was practical, demonstrating the electrical nature of nervus impulses. A galvanic (voltaic) prison cell uses the free energy released during a spontaneous redox reaction (\(ΔG < 0\)) to generate electricity. This type of electrochemical jail cell is often called a voltaic cell later on its inventor, the Italian physicist Alessandro Volta (1745–1827). In contrast, an electrolytic jail cell consumes electrical free energy from an external source, using information technology to crusade a nonspontaneous redox reaction to occur (\(ΔG > 0\)). Both types contain two electrodes, which are solid metals continued to an external circuit that provides an electrical connection between the ii parts of the system (Figure \(\PageIndex{ane}\)). The oxidation half-reaction occurs at one electrode (the anode), and the reduction half-reaction occurs at the other (the cathode). When the circuit is airtight, electrons menstruation from the anode to the cathode. The electrodes are as well continued by an electrolyte, an ionic substance or solution that allows ions to transfer between the electrode compartments, thereby maintaining the system's electrical neutrality. In this section, we focus on reactions that occur in galvanic cells.
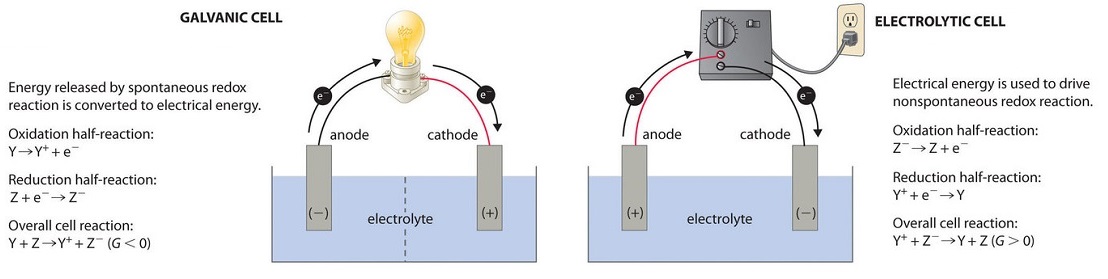
Voltaic (Galvanic) Cells
To illustrate the bones principles of a galvanic cell, let'south consider the reaction of metallic zinc with cupric ion (Cu2 +) to requite copper metal and Zn2 + ion. The balanced chemical equation is equally follows:
\[\ce{Zn (south) + Cu^{two+} (aq) \rightarrow Zn^{2+} (aq) + Cu(southward)} \label{twenty.3.4}\]
We can cause this reaction to occur by inserting a zinc rod into an aqueous solution of copper(Ii) sulfate. As the reaction proceeds, the zinc rod dissolves, and a mass of metal copper forms. These changes occur spontaneously, but all the energy released is in the class of oestrus rather than in a form that tin be used to do work.
This same reaction tin be carried out using the galvanic cell illustrated in Effigy \(\PageIndex{3a}\). To gather the cell, a copper strip is inserted into a beaker that contains a 1 Thou solution of \(\ce{Cu^{2+}}\) ions, and a zinc strip is inserted into a dissimilar beaker that contains a one M solution of \(\ce{Zn^{ii+}}\) ions. The two metal strips, which serve as electrodes, are connected by a wire, and the compartments are connected by a table salt bridge, a U-shaped tube inserted into both solutions that contains a concentrated liquid or gelatinous electrolyte. The ions in the salt span are selected and then that they do not interfere with the electrochemical reaction by beingness oxidized or reduced themselves or by forming a precipitate or complex; commonly used cations and anions are \(\ce{Na^{+}}\) or \(\ce{K^{+}}\) and \(\ce{NO3^{−}}\) or \(\ce{SO4^{2−}}\), respectively. (The ions in the common salt bridge practise not have to be the same as those in the redox couple in either compartment.) When the circuit is airtight, a spontaneous reaction occurs: zinc metal is oxidized to \(\ce{Zn^{2+}}\) ions at the zinc electrode (the anode), and \(\ce{Cu^{two+}}\) ions are reduced to \(\ce{Cu}\) metallic at the copper electrode (the cathode). As the reaction progresses, the zinc strip dissolves, and the concentration of \(\ce{Zn^{2+}}\) ions in the solution increases; simultaneously, the copper strip gains mass, and the concentration of \(\ce{Cu^{ii+}}\) ions in the solution decreases (Figure \(\PageIndex{3b}\)). Thus we accept carried out the aforementioned reaction every bit we did using a single beaker, simply this fourth dimension the oxidative and reductive half-reactions are physically separated from each other. The electrons that are released at the anode flow through the wire, producing an electric electric current. Galvanic cells therefore transform chemical energy into electrical energy that can then be used to practise piece of work.
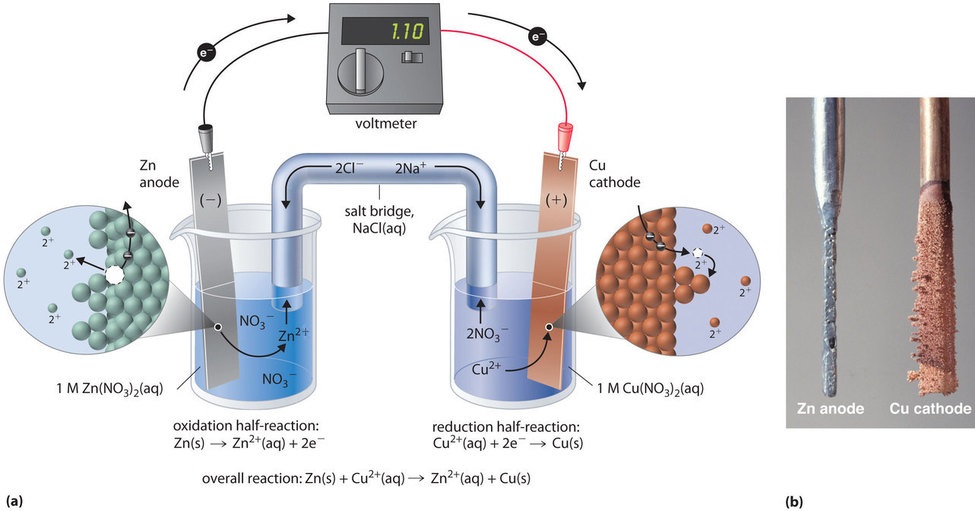
The electrolyte in the salt span serves ii purposes: it completes the circuit past carrying electrical accuse and maintains electrical neutrality in both solutions past allowing ions to drift between them. The identity of the salt in a common salt bridge is unimportant, as long as the component ions practise not react or undergo a redox reaction under the operating conditions of the prison cell. Without such a connectedness, the total positive accuse in the \(\ce{Zn^{2+}}\) solution would increase as the zinc metal dissolves, and the total positive charge in the \(\ce{Cu^{ii+}}\) solution would decrease. The salt bridge allows charges to exist neutralized by a period of anions into the \(\ce{Zn^{two+}}\) solution and a flow of cations into the \(\ce{Cu^{2+}}\) solution. In the absence of a table salt bridge or some other similar connexion, the reaction would apace stop because electrical neutrality could not be maintained.
A voltmeter can be used to measure the difference in electrical potential betwixt the two compartments. Opening the switch that connects the wires to the anode and the cathode prevents a current from flowing, so no chemical reaction occurs. With the switch closed, even so, the external circuit is airtight, and an current tin can flow from the anode to the cathode. The potential (\(E_{cell}\)) of the cell, measured in volts, is the difference in electrical potential betwixt the 2 half-reactions and is related to the energy needed to move a charged particle in an electric field. In the cell we have described, the voltmeter indicates a potential of one.10 Five (Figure \(\PageIndex{3a}\)). Because electrons from the oxidation half-reaction are released at the anode, the anode in a galvanic jail cell is negatively charged. The cathode, which attracts electrons, is positively charged.
Not all electrodes undergo a chemical transformation during a redox reaction. The electrode tin can exist made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does non announced in the overall electrochemical reaction. This phenomenon is illustrated in Example \(\PageIndex{1}\).
A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. An electrolytic cell consumes electrical free energy from an external source to drive a nonspontaneous chemical reaction.
Example \(\PageIndex{one}\)
A chemist has constructed a galvanic jail cell consisting of two beakers. One beaker contains a strip of tin immersed in aqueous sulfuric acrid, and the other contains a platinum electrode immersed in aqueous nitric acid. The two solutions are connected past a salt bridge, and the electrodes are connected past a wire. Current begins to flow, and bubbles of a gas appear at the platinum electrode. The spontaneous redox reaction that occurs is described by the following counterbalanced chemic equation:
\[\ce{3Sn(s) + 2NO3^{-}(aq) + 8H^{+} (aq) \rightarrow 3Sn^{2+} (aq) + 2NO (g) + 4H2O (l)} \nonumber\]
For this galvanic cell,
- write the one-half-reaction that occurs at each electrode.
- indicate which electrode is the cathode and which is the anode.
- indicate which electrode is the positive electrode and which is the negative electrode.
Given: galvanic prison cell and redox reaction
Asked for: one-half-reactions, identity of anode and cathode, and electrode assignment equally positive or negative
Strategy:
- Identify the oxidation half-reaction and the reduction half-reaction. Then identify the anode and cathode from the half-reaction that occurs at each electrode.
- From the direction of electron flow, assign each electrode every bit either positive or negative.
Solution
A In the reduction half-reaction, nitrate is reduced to nitric oxide. (The nitric oxide would then react with oxygen in the air to form NOii, with its characteristic cherry-red-brown color.) In the oxidation half-reaction, metallic tin is oxidized. The one-half-reactions corresponding to the actual reactions that occur in the organization are equally follows:
reduction: \[\ce{NO3^{−} (aq) + 4H^{+}(aq) + 3e^{−} → NO(g) + 2H2O(l)} \nonumber\]
oxidation: \[\ce{Sn(southward) → Sn^{2+}(aq) + 2e^{−}} \nonumber\]
Thus nitrate is reduced to NO, while the tin electrode is oxidized to Sntwo +.
Because the reduction reaction occurs at the Pt electrode, it is the cathode. Conversely, the oxidation reaction occurs at the tin electrode, and then information technology is the anode.
B Electrons flow from the tin electrode through the wire to the platinum electrode, where they transfer to nitrate. The electric circuit is completed by the table salt span, which permits the diffusion of cations toward the cathode and anions toward the anode. Considering electrons flow from the can electrode, it must exist electrically negative. In contrast, electrons flow toward the Pt electrode, so that electrode must be electrically positive.
Exercise \(\PageIndex{1}\)
Consider a simple galvanic cell consisting of two beakers connected by a common salt bridge. 1 beaker contains a solution of \(\ce{MnO_4^{−}}\) in dilute sulfuric acid and has a Pt electrode. The other chalice contains a solution of \(\ce{Sn^{2+}}\) in dilute sulfuric acrid, also with a Pt electrode. When the ii electrodes are connected by a wire, current flows and a spontaneous reaction occurs that is described by the following balanced chemical equation:
\[\ce{2MnO^{−}4(aq) + 5Sn^{2+}(aq) + 16H^{+}(aq) \rightarrow 2Mn^{2+}(aq) + 5Sn^{4+}(aq) + 8H2O(l)} \nonumber\]
For this galvanic cell,
- write the half-reaction that occurs at each electrode.
- betoken which electrode is the cathode and which is the anode.
- indicate which electrode is positive and which is negative.
- Answer a
-
\[\begin{align*} \ce{MnO4^{−}(aq) + 8H^{+}(aq) + 5e^{−}} &→ \ce{Mn^{ii+}(aq) + 4H2O(l)} \\[4pt] \ce{Sn^{2+}(aq)} &→ \ce{Sn^{4+}(aq) + 2e^{−}} \cease{align*}\]
- Respond b
-
The Pt electrode in the permanganate solution is the cathode; the one in the tin solution is the anode.
- Respond c
-
The cathode (electrode in chalice that contains the permanganate solution) is positive, and the anode (electrode in beaker that contains the can solution) is negative.
Electrochemical Cells: https://youtu.exist/nyS1BQ2ZVIg
Constructing Cell Diagrams (Cell Notation)
Because information technology is somewhat cumbersome to describe whatever given galvanic jail cell in words, a more convenient notation has been developed. In this line notation, chosen a jail cell diagram, the identity of the electrodes and the chemical contents of the compartments are indicated by their chemic formulas, with the anode written on the far left and the cathode on the far right. Stage boundaries are shown by single vertical lines, and the table salt bridge, which has two phase boundaries, by a double vertical line. Thus the cell diagram for the \(\ce{Zn/Cu}\) jail cell shown in Figure \(\PageIndex{3a}\) is written as follows:
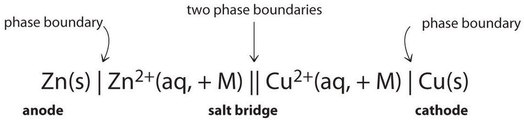
Galvanic cells can have arrangements other than the examples nosotros have seen so far. For example, the voltage produced past a redox reaction tin be measured more accurately using two electrodes immersed in a single chalice containing an electrolyte that completes the circuit. This arrangement reduces errors caused by resistance to the menstruum of charge at a boundary, called the junction potential. One example of this type of galvanic jail cell is as follows:
\[\ce{Pt(due south)\, | \, H2(g) | HCl(aq, \, one\,G)\,|\, AgCl(s) \,Ag(s)} \nonumber\]
This jail cell diagram does not include a double vertical line representing a salt bridge because there is no salt bridge providing a junction between ii unlike solutions. Moreover, solution concentrations have not been specified, then they are not included in the cell diagram. The half-reactions and the overall reaction for this cell are as follows:
cathode reaction:
\[\ce{AgCl (s) + east^{−} \rightarrow Ag(s) + Cl^{−}(aq)} \nonumber\]
anode reaction:
\[\ce{ 1/two H2(m) -> H^{+}(aq) + e^{-}} \nonumber\]
overall:
\[\ce{ AgCl(s) + i/2H2(m) -> Ag(s) + Cl^{-} + H^{+}(aq)} \nonumber\]
A unmarried-compartment galvanic cell volition initially exhibit the aforementioned voltage every bit a galvanic jail cell constructed using split compartments, but it will belch rapidly because of the direct reaction of the reactant at the anode with the oxidized member of the cathodic redox couple. Consequently, cells of this type are not specially useful for producing electricity.
Example \(\PageIndex{2}\)
Draw a jail cell diagram for the galvanic cell described in Example \(\PageIndex{1}\). The balanced chemic reaction is equally follows:
\[\ce{3Sn(southward) + 2NO^{−}3(aq) + 8H^{+}(aq) \rightarrow 3Sn^{2+}(aq) + 2NO(yard) + 4H2O(fifty)} \nonumber\]
Given: galvanic cell and redox reaction
Asked for: jail cell diagram
Strategy:
Using the symbols described, write the cell diagram start with the oxidation half-reaction on the left.
Solution
The anode is the tin strip, and the cathode is the \(\ce{Pt}\) electrode. Get-go on the left with the anode, we bespeak the phase boundary betwixt the electrode and the tin solution by a vertical bar. The anode compartment is thus \(\ce{Sn(s)∣Sn^{2+}(aq)}\). We could include \(\ce{H2SO4(aq)}\) with the contents of the anode compartment, simply the sulfate ion (equally \(\ce{HSO4^{−}}\)) does not participate in the overall reaction, and so it does not need to be specifically indicated. The cathode compartment contains aqueous nitric acid, which does participate in the overall reaction, together with the production of the reaction (\(\ce{NO}\)) and the \(\ce{Pt}\) electrode. These are written as \(\ce{HNO3(aq)∣NO(g)∣Pt(s)}\), with single vertical bars indicating the phase boundaries. Combining the two compartments and using a double vertical bar to bespeak the table salt bridge,
\[\ce{Sn(s)\,|\,Sn^{two+}(aq)\,||\,HNO3(aq)\,|\,NO(grand)\,|\,Pt_(s)} \nonumber\]
The solution concentrations were non specified, so they are not included in this cell diagram.
Practise \(\PageIndex{2}\)
Draw the jail cell diagram for the following reaction, assuming the concentration of \(\ce{Ag^{+}}\) and \(\ce{Mg^{2+}}\) are each i Chiliad:
\[\ce{Mg(south) + 2Ag^{+}(aq) \rightarrow Mg^{2+}(aq) + 2Ag(south)} \nonumber\]
- Answer
-
\[ \ce{ Mg(southward) \,|\,Mg^{ii+}(aq, \;1 \,M )\,||\,Ag^+(aq, \;ane\, M)\,|\,Ag(s)} \nonumber\]
Cell Diagrams: https://youtu.be/IKqOAfivem8
Summary
A galvanic (voltaic) cell uses the free energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical free energy from an external source to force a reaction to occur. Electrochemistry is the study of the relationship betwixt electricity and chemical reactions. The oxidation–reduction reaction that occurs during an electrochemical process consists of 2 half-reactions, one representing the oxidation procedure and one the reduction procedure. The sum of the half-reactions gives the overall chemical reaction. The overall redox reaction is counterbalanced when the number of electrons lost past the reductant equals the number of electrons gained by the oxidant. An electric electric current is produced from the flow of electrons from the reductant to the oxidant. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to bulldoze a nonspontaneous reaction. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic jail cell, electric energy is consumed to bulldoze a nonspontaneous redox reaction. Both types of cells use ii electrodes that provide an electrical connectedness between systems that are separated in space. The oxidative half-reaction occurs at the anode, and the reductive half-reaction occurs at the cathode. A salt bridge connects the separated solutions, allowing ions to migrate to either solution to ensure the organisation's electric neutrality. A voltmeter is a device that measures the flow of electric electric current between ii half-reactions. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electrical field. An electrochemical cell can be described using line notation called a cell diagram, in which vertical lines bespeak stage boundaries and the location of the common salt span. Resistance to the catamenia of charge at a purlieus is called the junction potential.
Source: https://chem.libretexts.org/Courses/University_of_California_Davis/UCD_Chem_002C/UCD_Chem_2C_(Larsen)/Text/02%3A_Electrochemistry/2.01%3A_Galvanic_Cells
Belum ada Komentar untuk "If a Galvanic Cell Is Prepared Correctly the Initial Multimeter Reading Be"
Posting Komentar